
FiteBac® Antimicrobial
Cavity Cleanser
New inventory coming soon.
FiteBac® Antimicrobial Cavity Cleanser removes debris in carious lesion preparations and penetrates exposed dentin tubules.
This activity allows restorative adhesives to tightly bind to the prepared dentin surfaces while acting on multiple microbes* within the restoration site.
* In vitro studies have shown FiteBac Antimicrobial Cavity Cleanser’s ability to act on the following microorganisms:
Streptococcus mutans 1
Actinomyces naeslundii 1
Lactobacillus acidophilus 2
Candida albicans 3
1. Gou YP, Li JY, Meghil MM, et al. Quaternary ammonium silane-based antibacterial and anti-proteolytic cavity cleanser. Dent Mater. 2018;34(12):1814-1827.
2. Daood U, Burrow MF, Yiu CKY. Effect of a novel quaternary ammonium silane cavity disinfectant on cariogenic biofilm formation. Clin Oral Investig. 2020;24(2):649-661.
3. Data on file.
How does FiteBac® work?
Si-Quat biocide covalently bound to a silane core containing acrylate functionality.
-
Acrylate functionality allows for
co-polymerization within several polymer systems
Patent protection until 2033.
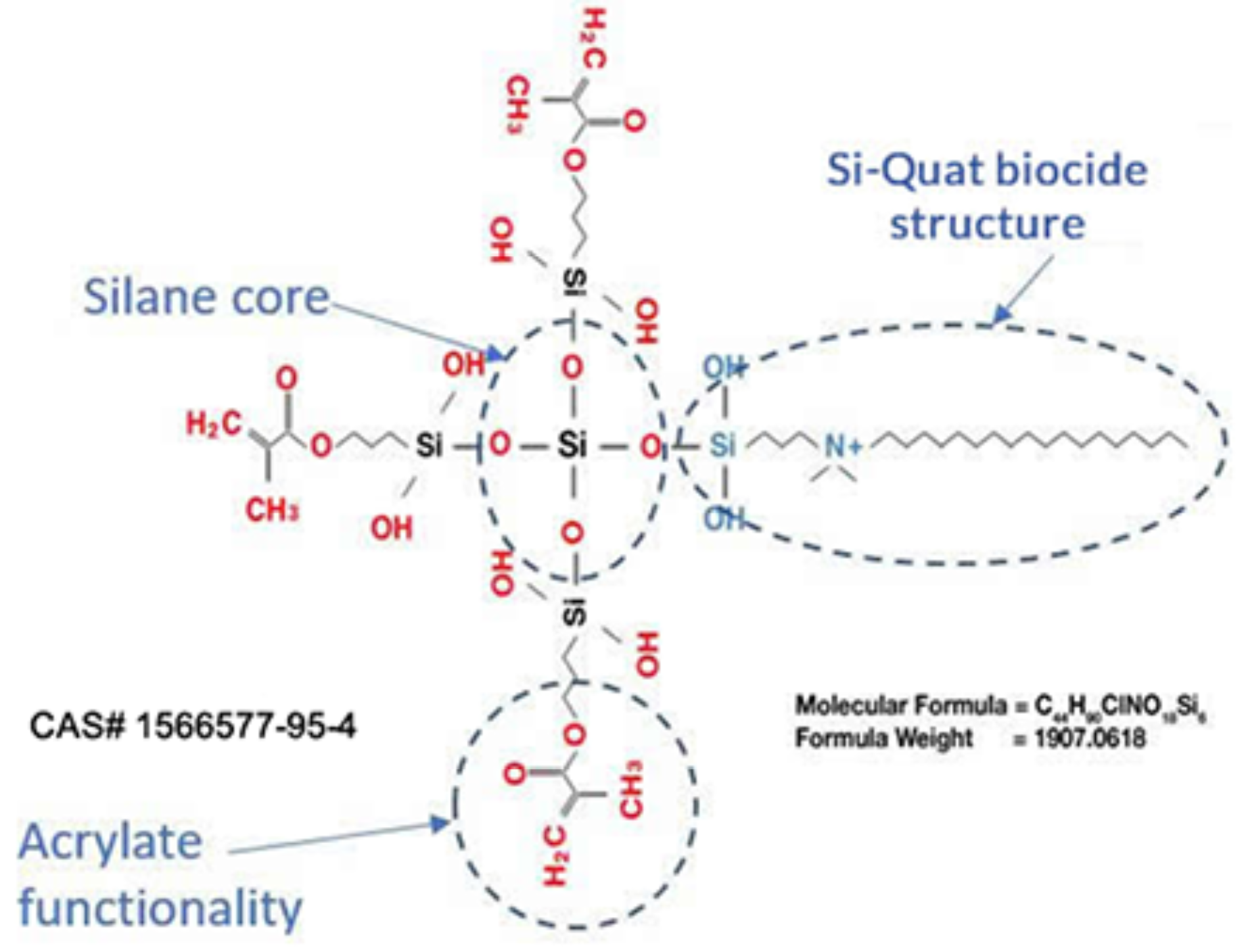
1. Gong SQ, Epasinghe J, Rueggeberg FA, et al. An ORMOSIL-containing orthodontic acrylic resin with concomitant improvements in antimicrobial and fracture toughness properties. PLoS One. 2012;7(8):e42355.
- Does not negatively impact adhesive bond strength to dentin2,3
- Excellent antimicrobial activity2,4
- Improves infiltration of adhesive monomers into acid etched dentin2
- Inhibition of MMP-9 and Cathepsin-K2,4
Patent protection until 2033.
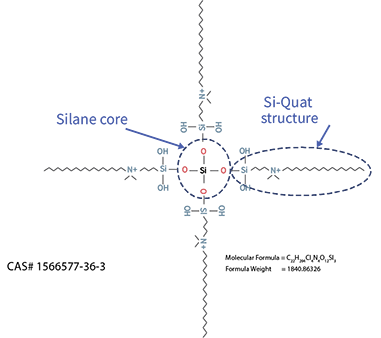
2. Gou YP, Li JY, Meghil MM, et al. Quaternary ammonium silane-based antibacterial and anti-proteolytic cavity cleanser. Dent Mater. 2018;34(12):1814-1827.
3. Daood D, Yiu CKY, Burrow MF, Niu LN, Tay FR. Effect of a novel quaternary ammonium silane cavity disinfectant on durability of resin-dentine bond. J Dent. 2017;60:77-86.
4. Umer D, Yiu CK, Burrow MF, Niu LN, Tay FR. Effect of a novel quaternary ammonium silane on dentin protease activities. J Dent. 2017;58:19-27.
Indications for Use: Intended for cleansing and moistening/re-wetting of cavity preparations
Development Status: Cleared for marketing in the United States
Target Audience: Prescription use for Dental Professionals
