FiteBac Technology, FiteBac’s chemical manufacturing arm, recently completed the development of antimicrobial additives for use in dental materials.

PRODUCT
DESCRIPTION
DATA SHEETS
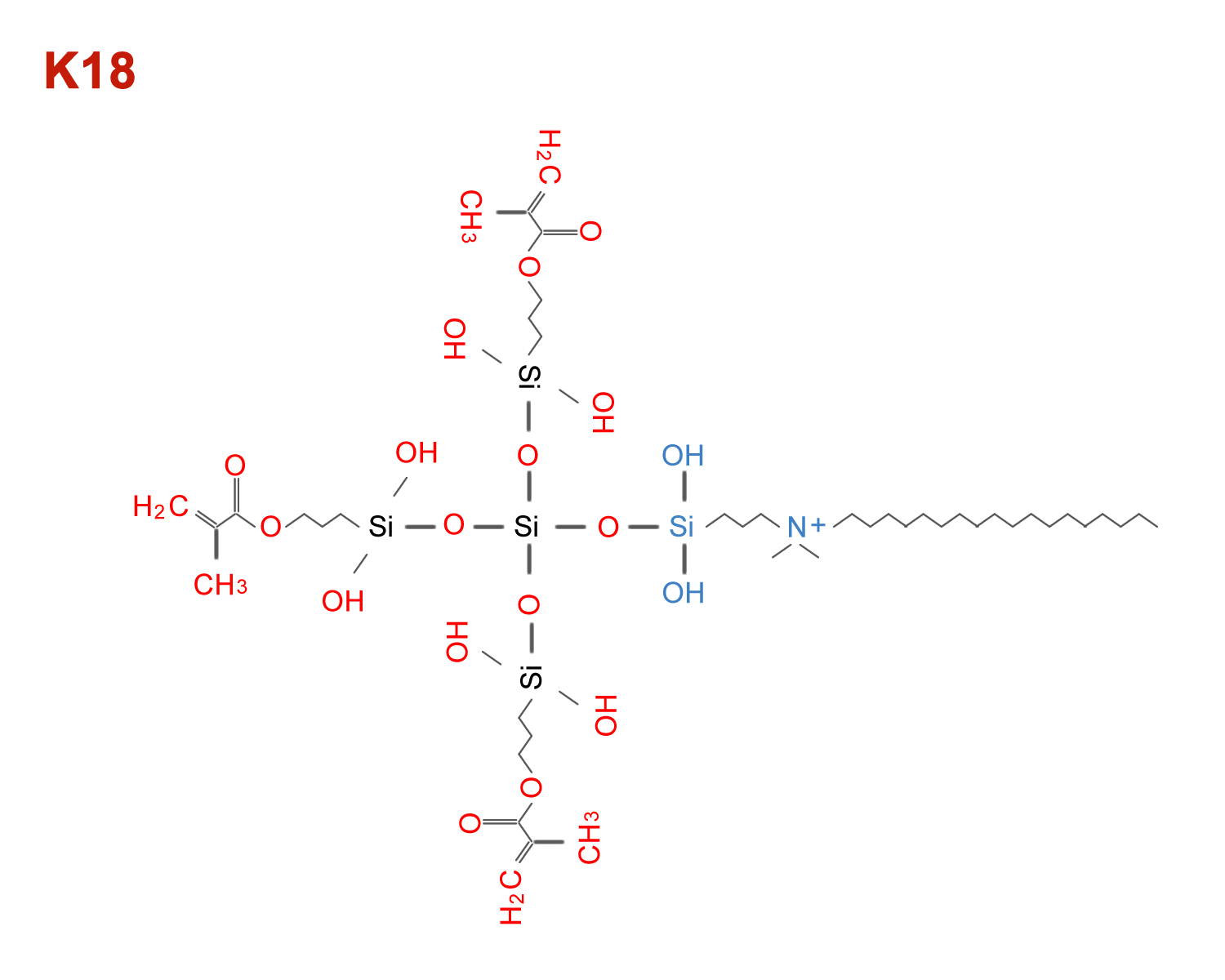
K18
K18 antimicrobial for use in adhesives, sealants, cements, endodontic sealer
K18M
K18 MMA for antimicrobial orthodontic acrylics, dentures, oral devices, partials
K18H
K18 HEMA antimicrobial for use in adhesives, sealants, resin systems
K18IBOA
K18 IBOA antimicrobial isobornyl acrylate for use in 3D printing resin systems
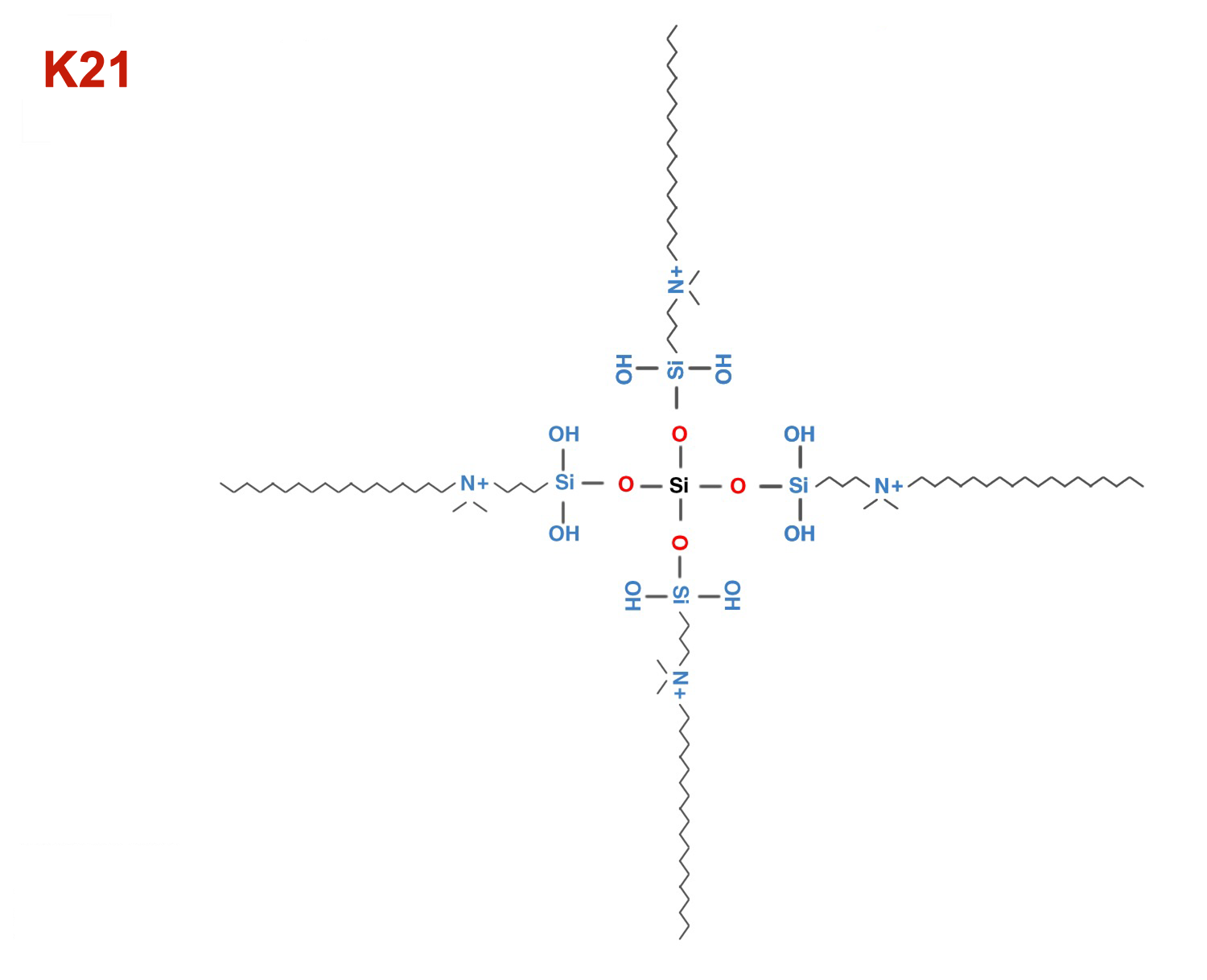
K21
K21 antimicrobial for cavity cleanser, sealants, mouth rinses
G30
G30 antimicrobial dental glass or silica for composites and resin systems with RI 1.530 and 0.7 micron
DS/SDS
GI
GI antimicrobial reactive glass for RMGI (Resin – Modified Glass Ionomer) cements
DS/SDS
G41
G41 antimicrobial dental glass or silica for composites and resin systems with RI 1.541 and 0.7 micron
DS/SDS
G50
G50 antimicrobial dental glass or silica for composites and resin systems on AEROSIL OX 50 (Silica)
DS/SDS
G55
G55 antimicrobial dental glass or silica for composites and resin systems with RI 1.555 and 0.7 micron
DS/SDS
RD70
RD70 antimicrobial silica with 17.5% K21 for silicone fluids for silicon mouth guards, resin systems, 15 micron
DS/SDS
S171
S171 advanced antimicrobial nanoparticle silsesquioxane with K18.5 methacrylate for use in all resin systems 400-600nm
DS/SDS
S1711
S1711 advanced antimicrobial nanoparticle silsesquioxane with K18 methacrylate and epoxy for use in all resin systems 400-600nm
DS/SDS
FiteBac Chemical K18 Biocompatibility Letter
Guidance for Potential Manufacturers
FDA regulatory compliance for cytotoxicity studies.
FiteBac Platform Chemistry
This video explains FiteBac’s antimicrobial technology and how it functions on a molecular level.
FiteBac’s LEAD ANTIMICROBIAL MOLECULES
Medical Device Master File Certificate
K-18 QAMS and K-21 QAS
FiteBac can provide access to published research that proves the effectiveness of our antimicrobial agents. They are already present in two FDA-cleared devices which others can cite as predicate devices to speed up FDA-clearance navigation.
Applications
FiteBac’s patented antimicrobial molecules are present in multiple dental devices that are cleared by the FDA and they are available for use in various areas of dentistry, such as:
Adhesives
Sealants
Cements
Endodontic Sealant
Mouth Rinse
Antimicrobial
Dental Glass
Silica
Resins
Crowns
Dentures
Implants
Silicone Mouth Guards
Composites
Night Guards
Retainers
Orthodontic Appliances
Resin-Modified Glass Ionomers